Chronic Lower Back Pain (CLBP) Market and Emerging Therapies

Chronic low back pain (CLBP) is defined as pain that persists for 12 weeks or longer, even after an initial injury or underlying cause of acute low back pain has been treated. Low back pain is very common and at one point everyone may have faced this problem. The exact cause of lower back pain is unknown. Lower back pain that is long-term (for more than 3 months) is called chronic low back pain, this condition might originate from an injury, disease, or stress on different structures of the body, and pain may vary significantly and maybe felt as bone pain, nerve pain, or muscle pain.
Chronic low back pain (CLBP) Market:
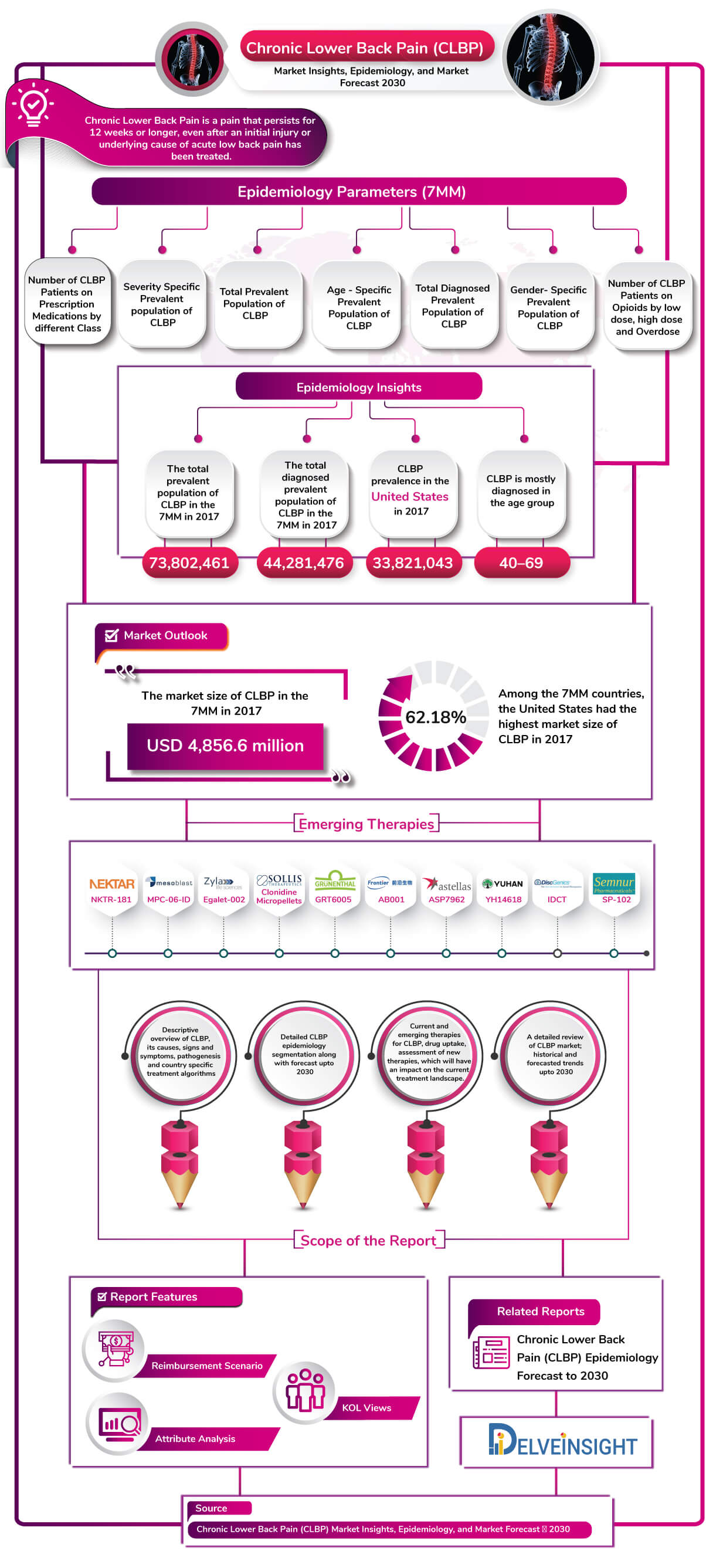
- According to the latest analysis, the total Prevalent Population of Chronic low back pain (CLBP) in the 7MM was found to be 73,802,461 in 2017.
- The estimates show a higher prevalence of chronic low back pain (CLBP) in the United States with 33,821,043 cases in 2017.
- Among the EU5 countries, the United Kingdom had the highest diagnosed prevalent population of CLBP with 3,517,443 cases, followed by Germany and then France. On the other hand, Spain had the lowest diagnosed prevalent population.
- The market size of CLBP in the 7MM was USD 4.8 billion in 2017.
This market report explores the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Chronic low back pain (CLBP) therapies, historical and forecasted market of Chronic low back pain (CLBP), and many more topics.
OR
Chronic low back pain (CLBP) Emerging Therapies:
Egalet-002 (Egalet Corporation), is a pure opioid that acts as opioid mu and kappa agonist; opioid delta agonists. The US FDA has granted Fast Track Designation for this drug. Eaglet Corporation has filed the NDA for this drug candidate. Egalet Corporation initially anticipated the NDA submission of Egalet-002 in 2019. Although the company completed phase III studies for Egalet-002 with proven efficacy, it has determined to delay indefinitely the previously-announced anticipated 2019 filing date for the Egalet-002 NDA, unless the availability of a partner to share the cost. The company is actively seeking partners for Egalet-002 as well as other product candidates.
(MPC-06-ID; Mesoblast) MPC-06-ID also known as Rexlemestrocel-L is a Mesoblast's proprietary allogeneic mesenchymal precursor cell (MPC) product candidate, currently in the late stage of development for the treatment of chronic low back pain caused by disc degeneration (CLBP). It is being developed for patients who have exhausted conservative treatment options, may have failed epidural steroid injections, and have no further treatment option other than invasive and costly surgical interventions. This drug works as a “Cell Replacement: Proteoglycan & collagen stimulant”.
Xtampa (Xtampza ER/ oxycodone) is a semisynthetic opioid analgesic derived from thebaine in Germany in 1917. It is currently indicated as an immediate release product for moderate-to-severe pain and as an extended-release product for chronic moderate-to-severe pain requiring continuous opioid analgesics for an extended period. It is used for the management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Collegium has developed a novel, patented, abuse-deterrent technology platform, DETERx, which provides extended-release drug delivery while safeguarding against common methods of abuse and tampering including crushing, chewing, and heating and injecting. Xtampza ER is its first product that is utilizing the DETERx technology platform.